PHYSIOMESH (recalled May 26, 2016)
Andrews & Thornton is reviewing and accepting cases for patients with ETHICON PHYSIOMESH™ hernia mesh who have required surgical replacement because of pain or hernia recurrence.
PHYSIOMESH was first sold in 2010. Ethicon recalled PHYSIOMESH because of bad patient results from data from two independent registries. These European registries track the outcomes of large numbers of hernia mesh repair surgeries. Evidence from these registries has shown that PHYSIOMESH fails at an unreasonably high rate.
Additionally, in a scientific study published in 2016, patients implanted with PHYSIOMESH were contrasted against patients implanted with a competitive mesh. Within 6 months, 20% of PHYSIOMESH patients experienced a hernia recurrence while none did in the competitive group. The study was stopped prematurely due to safety reasons. The authors concluded:
“[R]esults from the enrolled patients indicate that the [PHYSIOMESH] system associated with significantly greater hernia recurrences and postoperative pain compared with the [competitive] system.”
More than one million hernia repairs are performed each year in the U.S. A hernia occurs when an organ, intestine or fatty tissue squeezes through a hole or a weak spot in the surrounding muscle. Hernias often occur at the abdominal wall. Often a hernia can be visible as an external bulge.
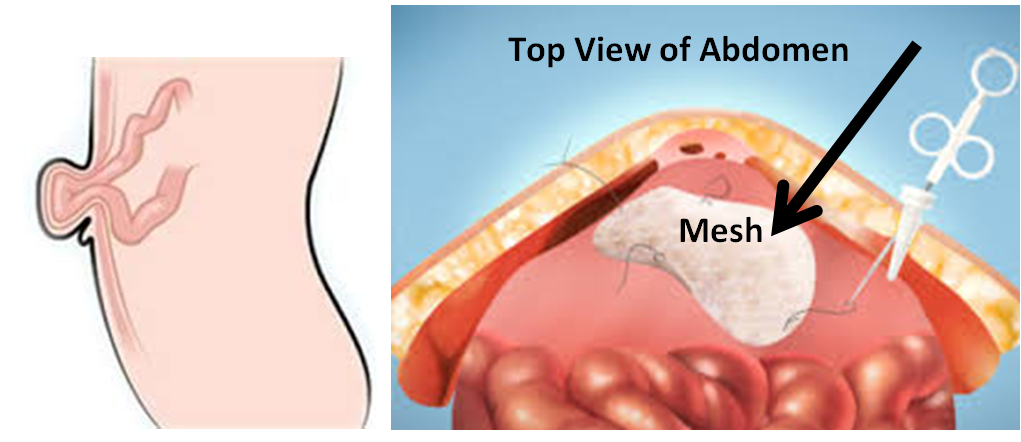
PHYSIOMESH is used in laparoscopic hernia repair. The surgeon makes three small incisions in the abdomen that allow the mesh and surgical tools into the openings to repair the hernia. PHYSIOMESH is unrolled once inside the abdomen and attached to the abdominal wall.
CONTACT OUR OFFICE FOR A FREE CASE EVALUATION!
Tel: (949) 748-1000 | Email: info@andrewsthornton.com







